
Get free, personalized support from OneSource—your source for help with navigating health insurance and Alexion treatment education.
Visit OneSource
A community of people who know what you’re going through can be key for patients and caregivers. We provide events near you as well as online webinars you can join from home.
Find an Event
This phone-based program connects patients and caregivers to people who share their experience with NMOSD. They offer assistance and support to those living with or caring for someone with NMOSD.
Enroll Now
Learn more about NMOSD at ENGAGE events. Hosted by a PEM, ENGAGE events are a great place to learn, connect with other adults patients, and more. Find a PEM by clicking on the button below and going to the Community Connections drop-down.
Connect With a PEM
To understand NMOSD relapses, learn about PREVENT clinical data, get prepared for SOLIRIS infusion treatment, and have support resources at a glance—the Make It SO Patient Brochure has you covered.
Download Brochure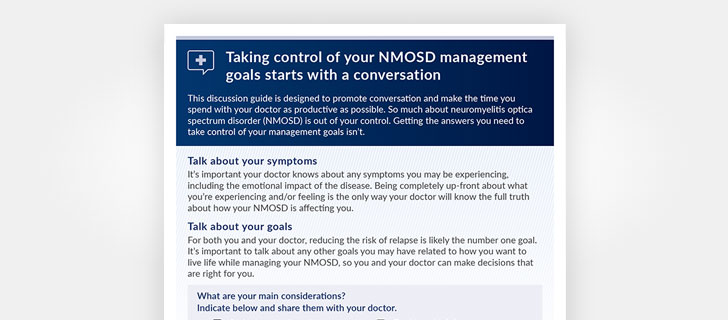
Our Doctor Discussion Guide can help you to keep track of your symptoms and questions so you can take charge of your treatment plan.
Download Guide
Your healthcare provider will give you a Patient Safety Card about the risk of meningococcal infection. Carry it with you at all times during treatment and for 3 months after your last dose of SOLIRIS. Your risk of meningococcal infection may continue for several weeks after your last dose of SOLIRIS. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
Download CardThe following organizations are not affiliated with Alexion, and Alexion is not responsible for information provided on their websites.
Supports programs and opportunities for improving the lives of adults with anti-AQP4 antibody-positive NMOSD. Visit guthyjacksonfoundation.org
Support for individuals with rare neuroimmune diseases like NMOSD and their families. Visit wearesrna.org
Provides advocacy and education to improve the lives of those affected by rare diseases. Visit rarediseases.org
One of the world’s leading rare disease patient advocacy organizations. Visit globalgenes.org
Generates global awareness and fundraising to help find a cure for NMOSD while providing support for both patients and their caregivers. Visit sumairafoundation.org
Sign up to get the latest news and information about anti-AQP4 antibody-positive NMOSD and how SOLIRIS may help.
*Required fields.
Your healthcare provider will give you a Patient Safety Card about the risk of serious meningococcal infection. Carry it with you at all times during treatment and for 3 months after your last dose of SOLIRIS. Your risk of meningococcal infection may continue for several weeks after your last dose of SOLIRIS. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
SOLIRIS may also increase the risk of other types of serious infections, including Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae. Certain people may be at risk of serious infections with gonorrhea. Certain fungal infections (Aspergillus) may occur if you take SOLIRIS and have a weak immune system or a low white blood cell count.
Tell your healthcare provider about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of SOLIRIS. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information and Medication Guide for SOLIRIS, including Boxed WARNING regarding serious meningococcal infections.
Your healthcare provider will give you a Patient Safety Card about the risk of serious meningococcal infection. Carry it with you at all times during treatment and for 3 months after your last dose of SOLIRIS. Your risk of meningococcal infection may continue for several weeks after your last dose of SOLIRIS. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
SOLIRIS may also increase the risk of other types of serious infections, including Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae. Certain people may be at risk of serious infections with gonorrhea. Certain fungal infections (Aspergillus) may occur if you take SOLIRIS and have a weak immune system or a low white blood cell count.
Tell your healthcare provider about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of SOLIRIS. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information and Medication Guide for SOLIRIS, including Boxed WARNING regarding serious meningococcal infections.